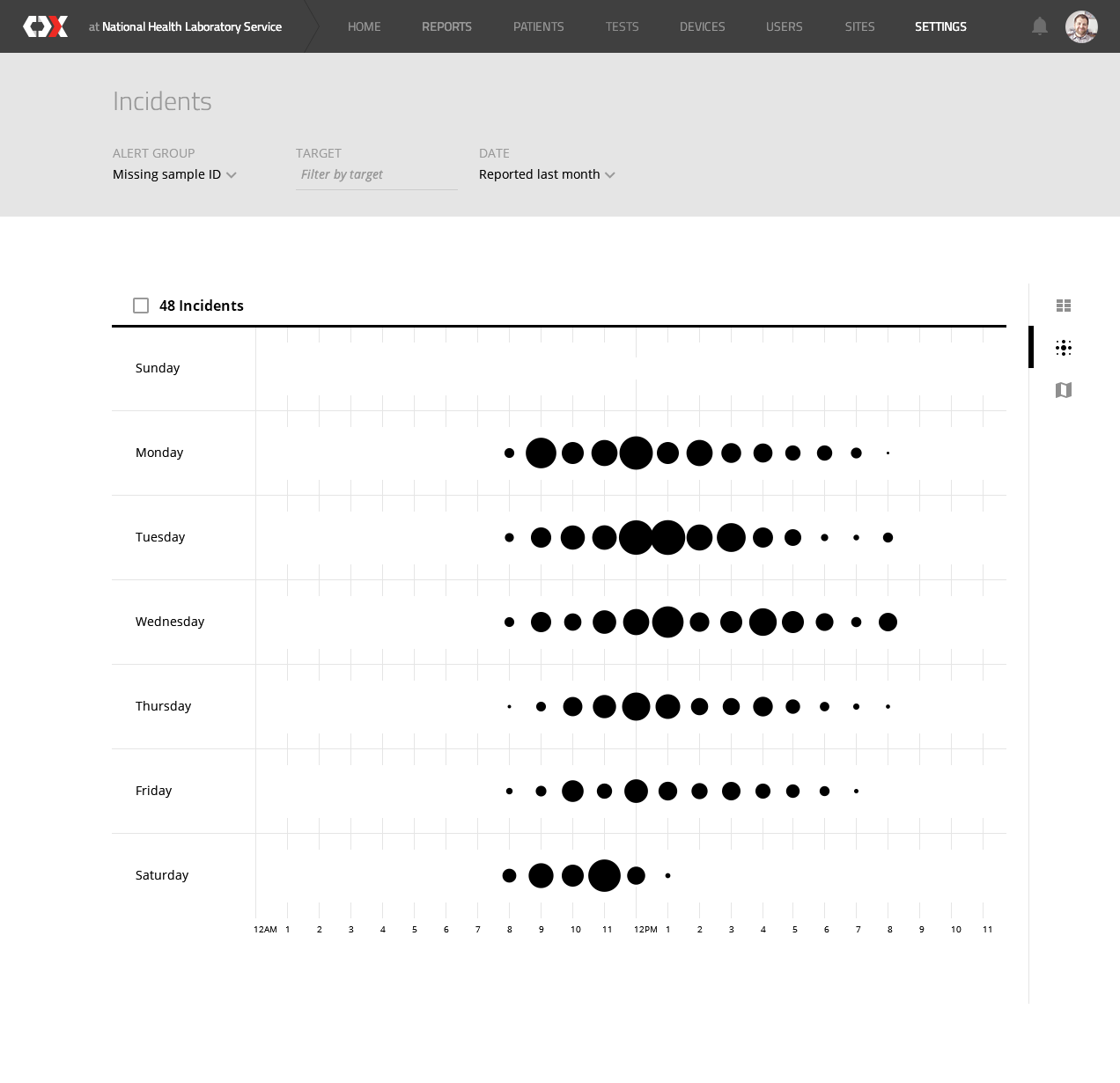
The CDx Platform is an aggregator of medical diagnostics data, allowing health workers and organisations to efficiently acquire, manage, store, and visualise diagnostic results and related data across devices, regardless of its manufacturer.
Ask for a demo



Data from any device in any format can be connected to CDx via a simple toolkit that transforms, adapts and protects the information.
Private information is stored securely. Flexible policies determine what is shared with different stakeholders at any aggregation level.
A rich API enables interoperability with external systems and extensions for new needs.